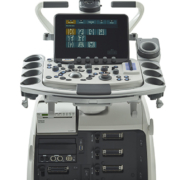
Premium ultrasound now FDA-cleared
Hitachi’s latest premium ultrasound system, the ARIETTA 850 received FDA 510(k) clearance last November and offers a collection of features designed to improve image quality, speed workflow, and extend the utility of ultrasound in radiology departments. ARIETTA 850 also supports the world’s first widely-available probe that uses capacitive micro-machined ultrasound transducers (CMUT) rather than piezoelectric crystals to transmit and receive the ultrasound signal. The new system builds upon the advanced capabilities of Hitachi’s ARIETTA family of ultrasound scanners, adding features like eFocusing, which removes the need for focal zone adjustments by dynamically focusing from the near to far-field of the image. It also expands interventional capabilities through a collection of features that work in conjunction with Hitachi’s Real-time Virtual Sonography fusion software to automatically perform fusion registration, compensate for needle flexion during biopsy procedures, and deliver real-time visual maps of estimated RF ablation zones. RVS (Real-time Virtual Sonography) offers superior real-time navigation for treatment, merging real-time ultrasound with previously acquired CT, MR images. 3D Sim-Navigator, an advanced function of RVS, provides assessment of the three-dimensional positional relationship between multiple electrode needles and the target lesion at the time of Radio Frequency Ablation (RFA). The E-field Simulator provides a pre-treatment simulation of the treatment area superimposed on the CT or MR, from the given location of the multiple electrodes.
Advancements in real-time RFA needle guidance can bring significant improvements to the treatment technique. The monitor arm and operating console of ARIETTA 850 have both been developed to provide a wide range of movement allowing ergonomic alignment so that the users’ comfort is maintained even during lengthy examinations. Additionally, Protocol Assistant, enabling prior examination protocol registration, promotes efficient workflow.
Supplier: Hitachi Medical Systems Europe Holding AG
Website: